The Physiology of Dynamic Thermometric Analysis of the Skin in Visceral Health & Disease
by Daniel Beilin, OMD, L.Ac., 2014

Overview
Internal dysfunction is reflected in skin surface temperature due to its connection to the autonomic nervous system. Therefore, various health and disease data can be evaluated by mapping skin temperature.
Abstract
Skin surface temperature may change in response to external and internal environmental stimuli. The skin is connected to internal organs, tissues, and glands via the autonomic nervous system. Internal physiologic abnormalities affect the regulation of skin surface temperature. External environmental temperatures may be controlled, and localized skin temperature may be monitored carefully according to known mappings (dermatomes and enterotomes). This enables the evaluation of visceral disorders. Regulation thermometry is a dynamic tool that enables the analysis of skin temperature before and after a fixed cooling period. Regulation thermometry may provide a monitor of visceral and glandular physiology and provides data about dysfunction that may precede or be caused by pathologic conditions.
Introduction
Thermoregulation is a function of human skin. Each skin function is mediated by specific cells or structures.(1) Keratinocytes provide durability, cohesion, and mechanical protection, and epidermal intercellular substances form an impermeable barrier. Melanocytes protect against damage from ultraviolet light, and Langerhans cells are important for the immune response. Merkel cells provide sensory function with nerve endings. Thermoregulation is provided by the extensive vascular supply, hair follicles, sweat glands, and adipose tissue of the skin. Vasoconstriction and vasodilation in the skin, controlled by the autonomic nervous system, contains functional physiologic information that is useful in medical assessment.
Skin Temperature Regulation
The skin is among the largest organs of the human body and is important in the regulation of the body’s core temperature. The skin may dissipate heat and becomes warm when the body must preserve body heat.(2) Skin temperature also is affected by the environment. In a cold environment, skin may lose heat and promote compensatory increased metabolism to maintain core temperature and prevent dermal tissue damage.(3)
In a warm environment, heat dissipation may occur from vasodilation of blood vessels in the skin and evaporation of excreted sweat.(4-6) Environmental temperature is sensed by skin thermosensors, which function to regulate core temperature. The relative contributions of core and skin temperature may vary to control body heat production and dissipation.(7) Heat dissipation by the skin is an important element of a feedback system that regulates core temperature.
Under moderate environmental conditions, skin temperature may depend primarily on the blood flow below the surface of the skin. In 1851, the French physiologist Claude Bernard suggested that the skin functions in the homeostasis of body temperature by controlling blood flow through cutaneous arterioles. Skin blood flow is affected by core, mean skin, and local skin temperature, mediated by reflex vasodilation and temperaturesensitive adenosine A1 receptors that cause vasoconstriction.(7-9) Capillary blood flow and arteriovenous shunting may be controlled by local temperature. In contrast with the voluntarily controlled muscles of the hands or feet, the muscles that control the cross-sectional area of arteries and arterioles are regulated by the autonomic nervous system, which responds involuntarily to nervous stimuli.(10)
Autonomic Nervous System
The autonomic nervous system is responsible for many vital functions and is not absolutely involuntary, because people can train themselves to control the effects of this system such as changes in hand temperature.(11) Until recently, it was impossible clinically to assess most autonomic nervous system functions reliably. Most autonomic nervous system functions involve the viscera, but objective quantifiable information about the normal and abnormal functions of the autonomic nervous system may be obtained from the dynamic behavior of skin temperature.
Models were developed to describe the interface between skin thermosensors and the central nervous system. Circulating blood is the main heat exchange agent in mammalian systems, and skin perfusion is the major route of heat dissipation.(12) Sensory information is integrated in the preoptic area of the anterior hypothalamus, and output signals affect core and skin temperature by cutaneous vasoconstriction, vasodilation, or arteriovenous shunting.
Thermoregulation of different areas of the body such as the forehead, back, abdomen, and breast, with and without stress, may show abnormalities before the development of more severe symptoms. These aberrations of thermoregulation may increase with disease progression.(13)
In summary, 1) capillary blood flow and arteriovenous shunting may be affected by local temperature, and 2) skin temperature regulation may be affected by reflex vasodilation. It is feasible to map information about local visceral status and visceral effects on shunting and reflex mechanisms to show value in dynamic studies of skin temperature changes in health and disease.
Reflexes
The architecture of the human body is dominated by the principle of segmentation, and all vertebrates are related to each other by similar neural connections. Viscerogenic reflexes can be understood and used from knowledge of the anatomy of segmentation and local neural networks. (14)
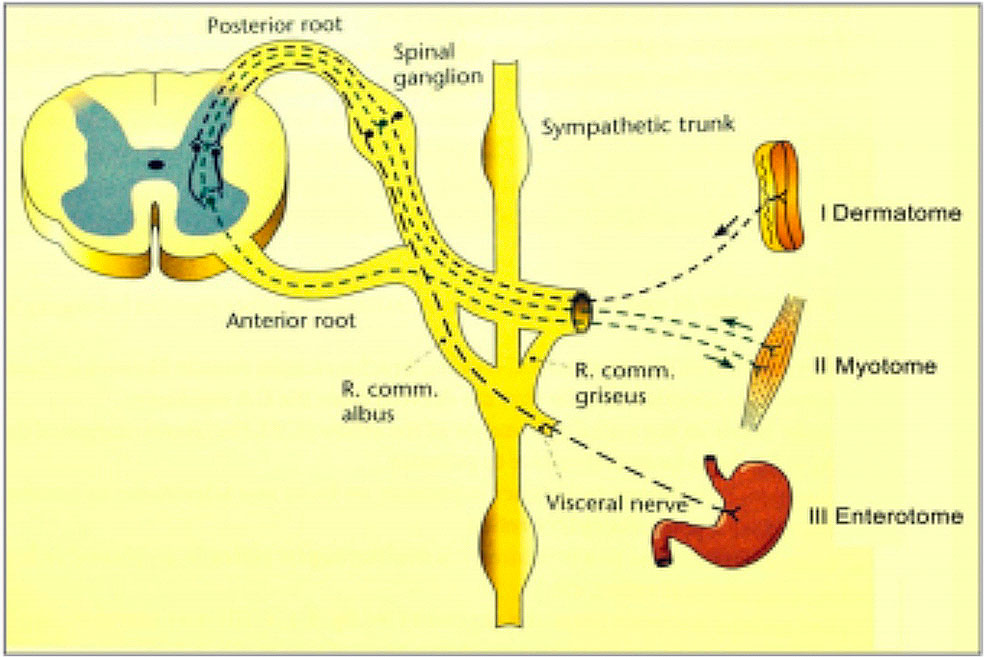
Figure 1. Reflex Relations Between Segmental Parts.
I. Multisynaptic reflex from dermatome to myotome.
II. Viscerogenic reflex from myotome to myotome.
III. Viscerogenic reflex from enterotome to myotome. * Modified from Hansen and Schliack.
Anatomic segments include dermatomes (integument), myotomes (muscles), sclerotomes (skeletal system), angiotomes (vascular system), enterotomes (viscera), or neurotomes (autonomic nervous system). A reflex occurs when a stimulus acts on a segment; a wave of excitation is conducted along an afferent pathway to the nerve center in the same segment, and a signal is sent back to the stimulated area along an efferent pathway (Figure 1).
Reflexes may be categorized as:
-
Proprioceptive: from 1 myotome to another,
-
Multisynaptic: from dermatome to myotome,
-
Miscerogenic: from enterotome to myotome, or
-
Viscerocutaneous: along afferent neurons through the sympathetic trunk to the posterior horn, through the lateral horn to the anterior horn, and sympathetic efferents to the integument and vasculature of the same segment. A viscerocutaneous reflex may elicit signs of pathology as an autonomic reflex in a large area of skin, even on an entire quadrant of the body.(15)
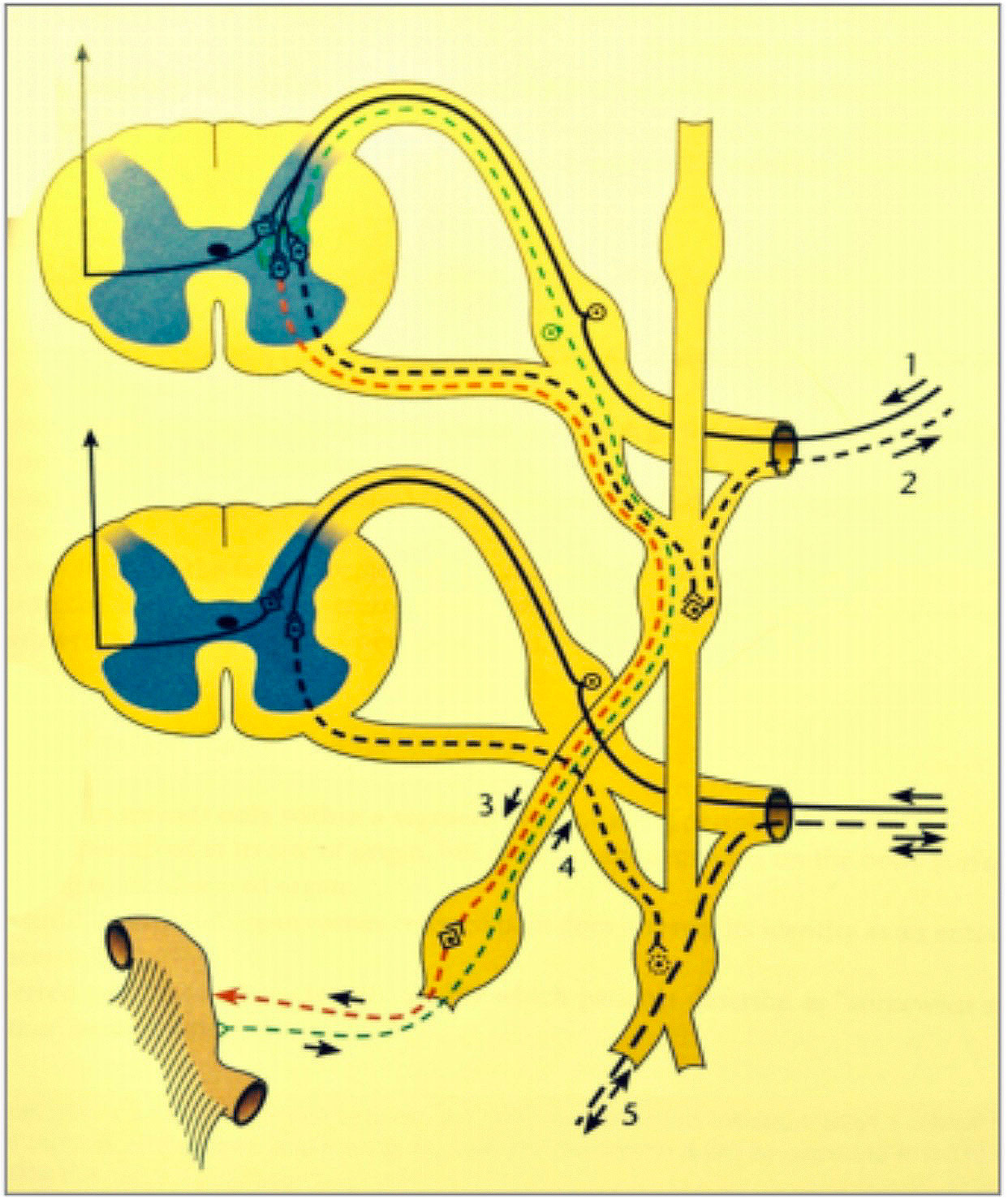
Figure 2. Reflexes between periphery and internal organs.
Line 1. Conduction of temperature sensations from the integument to the posterior horn of the spinal cord.
Line 2. Sympathetic neurons from the lateral horn to the integument with synapses in the sympathetic trunk.
Line 3. Sympathetic neurons from the lateral horn to the visceral organs that do not have synapses in the sympathetic trunk, but have synapses in the prevertebral ganglia.
Line 4. Afferent neurons from the visceral organs that conduct without synapses to the posterior horn.
Line 5. Peripheral axonal reflex between visceral organs and the integument, bypassing the spinal cord.
A cutivisceral reflex is initiated by stimulation of a dermatome or myotome and affects the enterotome belonging to the same segment. A viscerovisceral reflex originates in a diseased visceral organ and is conducted to another visceral organ. These reflexes cause nervous excitation of all parts of the segment, including the neurotome, which is the corresponding section of the spinal cord (Figure 1).
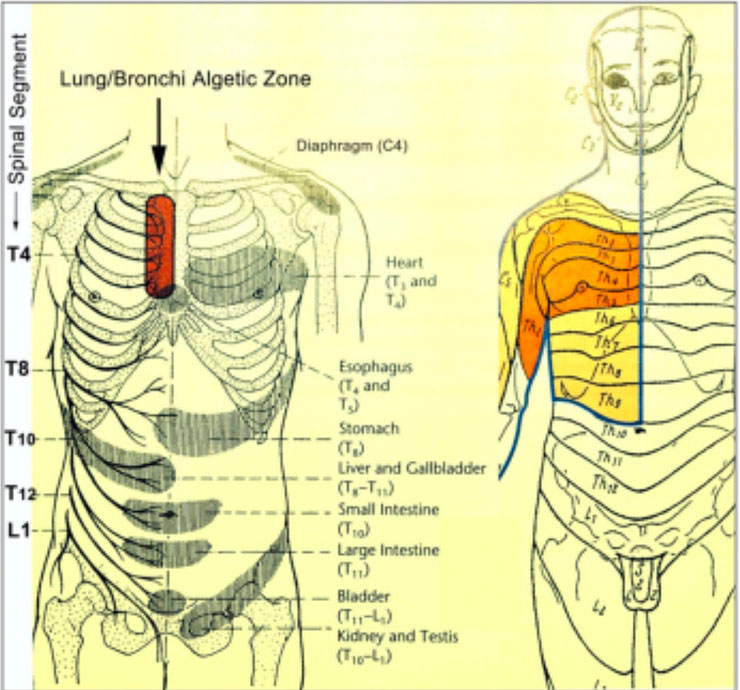
Figure 3. Algetic and Autonomic Reflex Projection of the Right Lung.
Head zone in the region of referred pain, corresponding to the maximum points of the affected dermatomes (myotomes, sclerotomes) (red).
A cutivisceral reflex is initiated by stimulation of a dermatome or myotome and affects the enterotome belonging to the same segment. A viscerovisceral reflex originates in a diseased visceral organ and is conducted to another visceral organ. These reflexes cause nervous excitation of all parts of the segment, including the neurotome, which is the corresponding section of the spinal cord (Figure 1).
A cutivisceral reflex is initiated by stimulation of a dermatome or myotome and affects the enterotome belonging to the same segment. A viscerovisceral reflex originates in a diseased visceral organ and is conducted to another visceral organ. These reflexes cause nervous excitation of all parts of the segment, including the neurotome, which is the corresponding section of the spinal cord (Figure 1).
Signal Projection
Visceral organs project signals that are indicative of disorders to sympathetic and parasympathetic nuclei. Therefore, visceral organs transmit information about disorders to the spinal cord segments from which sympathetic and parasympathetic innervations of organs are derived (Figure 2). The viscera use these neural pathways to transmit information about disorders to the dermatomes, myotomes, and sclerotomes that belong to the same segment, causing pain (algetic signs) and muscular tension (autonomic symptoms). The occurrence of palpable and visible changes elicited on the body surface by “projection” follows precise rules, and the quality and location of manifestations enable the deduction of the affected organ and diagnosis of problems in the body.(15) Sympathetic afferent neurons from the lungs and bronchi extend to the cells of the posterior horn of the spinal cord segments T2 to T5, and algetic signs are found on the trunk in the segments T2 to T5 (Figure 3).
Mechanism of Regulation Thermometry in Visceral Assessment
Sensitivity to changing skin temperature is a prominent feature of the skin thermoreceptors. The magnitude of the phasic response depends on the range within which the temperature is changed, in relation to the static response curve, usually being largest near the static maximum.(16) The size of the phasic response is affected by the rate of temperature change. Peak frequency increases nonlinearly with increasing rate of temperature change. Definite dynamic responses have been observed to rates of temperature change as low as 0.04oC, which is within the range observed to exert dynamic effects on heat production. (17-18)
In contrast, when a step change of skin temperature is applied, the receptor and autonomic responses are dissociated from each other (Cabanac, 1975); the transient surface receptor response ceases within 30 to 40 seconds and the autonomic response requires 10 minutes (Figure 4).(16-18) This discrepancy may become smaller when subcutaneous thermoreceptors are considered, and deeper layers of skin may have more persistent temperature changes in response to a step change on the surface.
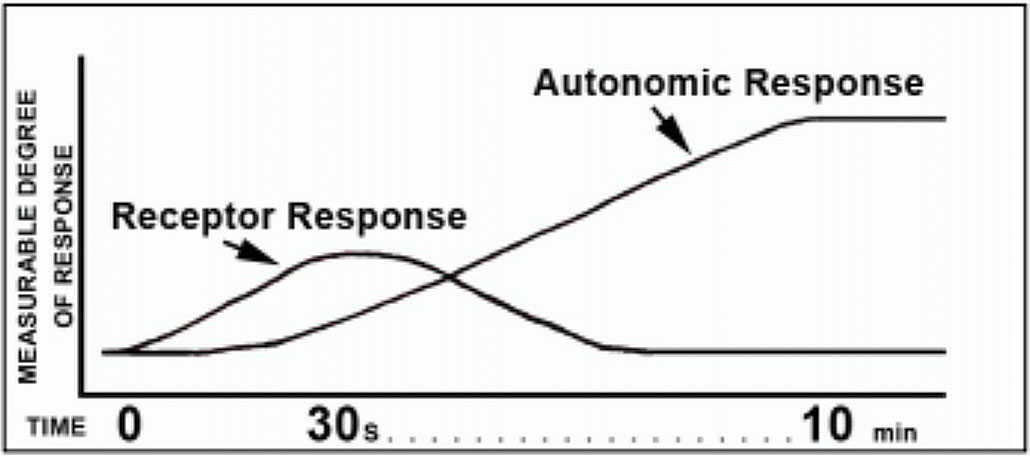
Figure 4. Differentiation of Receptor and Autonomic Response After Application of a Step Change in Skin Temperature.
The hypothalamic response delivers visceral information to the arteriolar smooth muscle and mediates muscle contraction or relaxation. Stabilization occurs after 10 minutes.
The autonomic response is used in regulation thermometry by taking a second set of temperature values at 10 minutes to maximize sampling of the autonomic bell curve. This may avoid elements of the central or peripheral nervous system that may not be involved in the visceral information pathway or may be mixed with other healthy responses to cool air exposure. By letting the 10 minutes elapse (time filtration), we maximize the information that originates from visceral and tissue signaling capacity and behavior.
In regulation thermometry, we use the glabella (prominence between the eyebrows) to construct the baseline because the vasomotor neurons in this region are minimally reactive to cold stimuli and this is the most thermally stable region on the human body.(19)
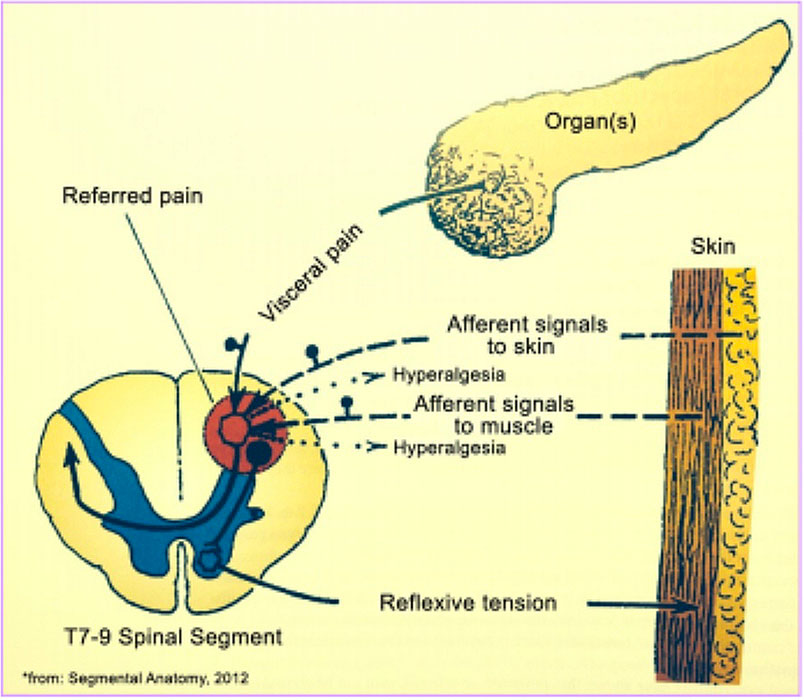
Figure 5. Development of Referred Pain.
Cumulative impulses from the viscera and sensory afferent neurons of a single segment may cause a field of irritation in the posterior horn. The brain initiates an immediate response (within several seconds) and the spinal cord initiates a slower but broader response that may be measured by temperature change of the skin in that segment.
Viscera
Control of blood flow in the viscera such as the liver, gut, and muscles occurs by continuous, dynamic opening and closing of blood vessels that may be affected by numerous factors. This vascular control is not caused by a fixed caliber of the arterioles. Blood flow through each organ is affected by the partial pressure of oxygen and carbon dioxide, glucose levels, and endocrine effects.(20) The impulse and effects of cool air stress may include clear effects on organ system metabolism and functional balancing mechanisms. These effects in a stable test environment (20oC to 22oC) may offset the normal responses to cool air in the segmental region. Head zones are areas of skin hyperalgesia that are associated with visceral disease.
Viscerocutaneous projection zones and the net effect can be measured on the skin in successive measurements according to the autonomic (primarily sympathetic and cholinergic) effects and resulting modulations to normal response.
References
(1) Bressler RS, Bressler CH. Functional anatomy of the skin. Clin Podiatr Med Surg. 1989;6(2):229-246.
(2) Page EH, Shear NH. Temperature-dependent skin disorders. J Am Acad Dermatol. 1988;18(5 pt 1):1003-1019.
(3) Bittel JH, Nonotte-Varly C, Livecchi-Gonnot GH, Savourey GL, Hanniquet AM. Physical fitness and thermoregulatory reactions in a cold environment in men. J Appl Physiol. 1988;65 (5):1984- 1989.
(4) Hayward JS, Eckerson JD, Collis ML. Thermoregulatory heat production in man: prediction equation based on skin and core temperatures. J Appl Physiol Respir Environ Exerc Physiol. 1977;42 (3):377-384.
(5) Nadel ER, Horvath SM, Dawson CA, Tucker A. Sensitivity to central and peripheral thermal stimulation in man. J Appl Physiol. 1970;29(5):603-609.
(6) Vogelaere P. Thermoregulatory nonshivering thermogenesis in men. Int J Biometeor. 1990;34(1):24-27.
(7) Jessen C. Thermal afferents in the control of body temperature. Chapter 7. In: Schönbaum E, Lomax P, eds. Thermoregulation: Physiology and Biochemistry. London, England: Pergamon Press; 1990
(8) Guyton AC. Body temperature, temperature regulation and fever. Chapter 73. In: Guyton AC, ed. Textbook of Medical Physiology. 8 th ed. City, State: Publisher; 1991
(9) Stojanov I, Proctor KG. Temperature-sensitive adenosinemediated vasoconstriction in the skin microcirculation. J Pharmacol Exp Ther. 1990;253(3):1083-1089.
(10) Abdel-Rahman TA, Collins KJ, Cowen T, Rustin M. Immunohistochemical, morphological and functional changes in the peripheral sudomotor neuro-effector system in elderly people. J Auton Nerv Syst. 1992;37(3):187-197.
(11) Ikemi A, Tomita S, Hayashida Y. Thermographical analysis of the warmth of the hands during the practice of self-regulation method. Psychother Psychosom. 1988;50(1):22-28.
(12) Boulant JA, Curras MC, Dean JB. Neurophysiological aspects of thermoregulation. Chapter 4. In: Wang LCH, ed. Advances in Comparative and Environmental Physiology. Vol. 4. Berlin, Germany: Springer-Verlag; 1989:117-160.
(13) Saitoh M, Hara F, Okumura H. Autonomic nervous activity in patients with chronic liver disease especially liver cirrhosis [in Japanese]. Nihon Ika Daigaku Zasshi. 1991;58(1):11-23.
(14) Hansen K, Schliack H. Segmental Innervation. Stuttgart, Germany: Thieme Verlag; 1962:411-xxx.
(15) Wancura-Kampik I. Segmental Anatomy. Munich, Germany: Elsevier Urban & Fischer Verlag; 2009.
(16) 16. Kenshalo X, Duclaux X. Title of chapter. In: Willis WD Jr, Coggeshall RE, eds. Sensory Mechanisms of the Spinal Cord. New York, New York: Springer; 1990:271-280 .
(17) Molinari HH, Kenshalo DR.. Effect of cooling rate on the dynamic response of cat cold units. Exp Neurol. 1977;55(3 pt 1):546- 555.
(18) Timbal J, Boutelier C, Loncle M, Bougues L. Comparison of shivering in man exposed to cold in water and in air. Pflugers Arch 1976;365(2-3):243-248.
(19) Hensel H. Thermoreception and Temperature Regulation (Monographs of the Physiological Society). London, England: Academic Press; 1981.
(20) Grayson J, Oyebola DD. The effect of catecholamines on intestinal glucose and oxygen uptake in the dog. J Physiol. 1983; 343:311-322.
Additional Reference: Brown AC, Brengelmann GL. The interaction of peripheral and central inputs in the temperature regulation system. Chapter 47. In: Hardy JD, Gagge AP, Stolwijk JAJ, eds. Physiological and Behavioral Temperature Regulation. Springfield, IL: Charles C. Thomas Publisher; 1970:684-702.